8 Biotech Stocks With Major Catalysts on the Horizon
Biotech stocks frequently make big moves on a single trial result or FDA ruling. These 8 drugmakers are worth watching amid important events on deck this summer.

Profit and prosper with the best of Kiplinger's advice on investing, taxes, retirement, personal finance and much more. Delivered daily. Enter your email in the box and click Sign Me Up.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered daily
Kiplinger Today
Profit and prosper with the best of Kiplinger's advice on investing, taxes, retirement, personal finance and much more delivered daily. Smart money moves start here.

Sent five days a week
Kiplinger A Step Ahead
Get practical help to make better financial decisions in your everyday life, from spending to savings on top deals.

Delivered daily
Kiplinger Closing Bell
Get today's biggest financial and investing headlines delivered to your inbox every day the U.S. stock market is open.

Sent twice a week
Kiplinger Adviser Intel
Financial pros across the country share best practices and fresh tactics to preserve and grow your wealth.

Delivered weekly
Kiplinger Tax Tips
Trim your federal and state tax bills with practical tax-planning and tax-cutting strategies.

Sent twice a week
Kiplinger Retirement Tips
Your twice-a-week guide to planning and enjoying a financially secure and richly rewarding retirement

Sent bimonthly.
Kiplinger Adviser Angle
Insights for advisers, wealth managers and other financial professionals.
Sent twice a week
Kiplinger Investing Weekly
Your twice-a-week roundup of promising stocks, funds, companies and industries you should consider, ones you should avoid, and why.

Sent weekly for six weeks
Kiplinger Invest for Retirement
Your step-by-step six-part series on how to invest for retirement, from devising a successful strategy to exactly which investments to choose.
We all know that investing in biotech stocks isn't for the faint of heart.
They tend to be pretty volatile, experiencing massive price surges and slumps. The reason for all these swings is simple – biotech stocks trade on catalysts.
Unlike, say, a steady consumer products firm, biotech stocks march to the drum of regulatory approval, the outcomes of trial data and major conference presentations. Often, stocks in the sector will zoom higher or sink lower on these pieces of information.
Then it becomes a waiting game until the next major catalyst.
For investors looking to play in biotech stocks, knowing these important dates is vital for success. The worst thing to do is get caught off-guard and not have an exit strategy if things suddenly get ugly for your investment.
While the start of the calendar year is often prime time for U.S. Food and Drug Administration (FDA) events and trial data to be released, the summer also heats up for drugmakers and biotech stocks. The next few months feature several major events for a number of biotechnology firms and their shareholders.
The key is knowing which events to watch.
Finding Prescription Drug User Fee Act (PDUFA) dates – which are deadlines for the U.S. FDA to review a company's drugs – and other info for these biotech catalysts can be a daunting task. Here, we'll point out several of these dates for investors to mark on their calendars.
Here are eight biotech stocks with major catalysts on the calendar in the next few months.
Data is as of May 18. Note: Any dates listed here are tentative and subject to change.

Bristol Myers Squibb
- Market value: $146.5 billion
- Timeline to watch: September 2021
With its purchase of Celgene in late 2019, Bristol Myers Squibb (BMY, $65.60) became a biotech and cancer-fighting machine. BMY has top cancer drugs like Opdivo, Revlimid, Yervoy and Pomalyst under its umbrella, and its oncology drugs now account for roughly 66% of its total revenues – or more than $27 billion last year. Any catalysts that deal with BMY's cancer portfolio are paramount to its bottom line.
Many recent events for BMY have focused on its blockbuster drug Opdivo. The treatment has been a mixed bag lately, with overall sales falling 3% year-over-year to $1.7 billion in the first quarter of 2021. This follows a similar decline in fiscal 2020. Merck's (MRK) own wonder cancer drug Keytruda has taken much of Opdivo's thunder and has seen sales explode, jumping 19% year-over-year in the first quarter of 2021.
Drugmakers can often pick up incremental sales, push patent expiration and breathe new life into older drugs with expanded label coverage. With Opdivo sales falling as Keytruda gains more market share, pivoting the drug into other cancer-fighting uses is critical to BMY getting more sales into its coffers.
And with Keytruda sales rising fast, the biotech stock needs some major wins to keep the drug a powerhouse. It could get those this summer, with the FDA expected to issue a decision on using Opdivo as an adjuvant therapy in patients with muscle-invasive urothelial carcinoma by Sept. 3, 2021.
Guggenheim analyst Seamus Fernandez, who maintains a Buy rating on BMY, is upbeat about Opdivo sales. Physician checks that showed "solid early uptake" for the drug and the "combined use of Opdivo plus Yervoy and Opdivo plus Cabometyx will expand Opdivo sales significantly," he says. Because of this, Guggenheim estimates an additional $3.5 billion in Opdivo sales between 2025 and 2028, with revenue peaking at around $11 billion by the time the drug's patent expires in 2028.
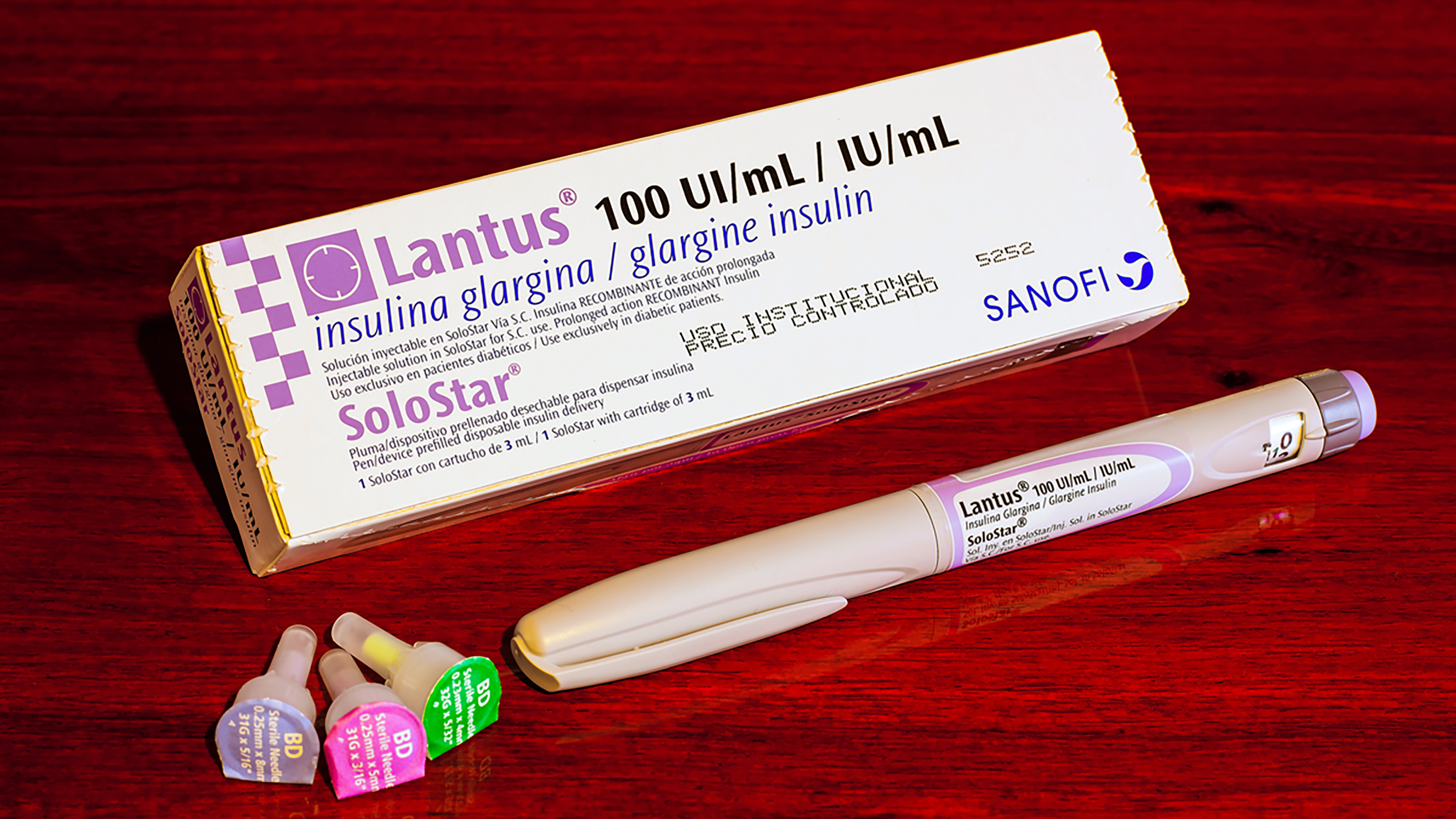
Sanofi
- Market value: $132.6 billion
- Timeline to watch: August 2021
French drug giant Sanofi (SNY, $53.47) has been in the news lately for its COVID-19 efforts, reporting positive Phase 2 clinical trial data alongside GlaxoSmithKline (GSK). SNY is late to the vaccine party, though, and isn't projecting regulatory approval until at least the fourth quarter of 2021. However, there are other drug events on the calendar this summer that could serve as a catalyst for the biotech stock.
Chief of which is the FDA's Aug. 18 review date for Sanofi's avalglucosidase alfa, its enzyme replacement therapy (ERT) for Pompe disease. The review was initially scheduled for May 18, but was extended by three months.
Pompe disease is a rare genetic disorder that is caused when your body is unable to develop a protein that breaks down complex sugars called glycogen for energy. These sugars will then build up and eventually damage various organs and muscles. Typically, the disease affects children, but adults can get it as well.
Currently, there is no cure for Pompe disease, but there are treatments. Enzyme replacement therapy (ERT) is allowing doctors to basically inject the missing protein into a patient's body to break down the excess sugar. Sanofi owns the only ERT drug on the market, with FDA-approved Lumizym costing nearly $300,000 per year. (Lumizym is marketed as Myozyme in Europe.)
The second treatment set for review later this summer would be the new standard of care for Pompe disease and feature an advanced mechanism of action for getting the protein into muscles. The FDA already gave breakthrough therapy and fast-track designations to the drug last fall based on its trial data and need, which allows the company to hold patents for longer and move more quickly through the approval process.
While Sanofi has been hush on pricing for the new drug if approved, the firm pulled in $1.1 billion in sales from its ERT medications in 2020. Given that it's the world leader, another high-cost drug in its arsenal will boost those sales further.

Alkermes
- Market value: $3.5 billion
- Timeline to watch: June 2021
While its name might not sound familiar, Alkermes (ALKS, $21.88) is no slouch in the biotech world. The firm already markets two drugs in the U.S. – Aristada for schizophrenia and Vivitrol for alcohol and opioid addiction. Those drugs continue to work well for the firm. Revenues for the two drugs accounted for more than half of the $251 million in sales ALKS brought in during the first quarter.
On June 1, Alkermes could add another neurological drug to its umbrella.
After accepting Alkermes' resubmission of the New Drug Application for ALKS 3831 earlier this year – required due to the FDA's request last November that conditions at one of the company's facilities be resolved – the regulatory agency is expected to issue its decision on the treatment.
The drug is designed to treat adults that have been diagnosed with schizophrenia and bipolar I disorder. Featuring a new novel compound, ALKS could have another hit on its hand.
Analysts at research firm Prophecy Market Insights peg the global schizophrenia drug market to be worth around $9.8 billion in 2030 – up from $7.5 billion in 2020 – and ALKS could be positioned to take a piece of the pie. Given that the FDA committee addressed the safety and weight gain concerns in the resubmission, approval for ALKS 3831 seems imminent.
And while the approval would generate sales, the real win is the momentum it could provide ALKS throughout the year. The firm recently partnered with Merck for a combo-therapy using Keytruda to treat ovarian cancer and recent activist activity from Sarissa Capital Management helped drive the biotech stock up 18% last month.
Another FDA approval could help the biotech stock break out and reward shareholders.
Provention Bio
- Market value: $467.1 million
- Timeline to watch: May 2021
One man's trash is another man's treasure. In the biotech world, that happens when one firm can't make a therapy work. Sometimes, they'll sell all the research and intellectual property (IP) rights for the drug on the cheap to another developer willing to pick up the pieces.
Which brings us to Provention Bio (PRVB, $7.37).
Back in 2010, Eli Lilly (LLY) – one of the largest global producers of insulin – and MacroGenics (MGNX) looked to team up to produce a type 1 diabetes medicine, Teplizumab. However, after Phase 3 trial data failed to meet the goals of the study, LLY shelved the project.
But in 2018, Provention picked up the drug on the cheap from MGNX and has been working to overcome some of the issues that it previously faced. That hasn't always gone to plan, however.
Earlier this year, an FDA committee meeting showed the regulatory agency found significant "deficiencies" between the pharmacokinetic and pharmacodynamic profiles generated by Eli Lilly and those manufactured by Provention Bio, and requested additional data.
Provention said this was due to the differences in contract manufacturers for the drug, but agreed to work closely with the FDA to "address its additional data requirement." On May 27, PRVB is expected to find out if an FDA advisory committee will recommend the approval of Teplizumab for the treatment of type 1 diabetes.
That could be a game changer.
For starters, the drug is designed to delay or prevent type 1 diabetes symptoms in certain at-risk individuals. That's huge and could upend the way we treat diabetes. It could also send billions into the young biotech's coffers.
Secondly, it's a make-or-break moment for Provention Bio itself. The firm had about $207 million in cash on its balance sheet at the end of the first quarter. If the FDA advisory committee recommends denying the application for Teplizumab, it will likely mean another round of costly trials for PRVB. The question is whether or not it has the cash to keep that going considering its other drugs in development stages.
While the biotech stock isn't an outright gamble, it is a risky one, given that the May PDUFA date is critical for Provention Bio's future.

Myovant Sciences
- Market value: $2.1 billion
- Timeline to watch: June 2021
Women's health is one of the fastest growing market segments for drugmakers. For far too long, many women-specific health needs have gone unmet from drug manufacturers. That's been changing in recent years, with a variety of drugs being launched to cover these concerns. U.K. biotech stock Myovant Sciences (MYOV, $22.60) is one such firm.
The key to that focus is MYOV compound Relugolix. The drug has already been approved for treating certain prostate cancers. But the firm has been able to pivot the drug in certain combination therapies for other health needs. In this case, uterine fibroids.
It's estimated that more than 171 million women across the globe suffer from the non-cancerous tumors. And they're a big problem with heavy and prolonged menstrual bleeding, anemia and pelvic pain.
Right now, the treatment options are fairly limited, and include hysterectomies and progestin-releasing intrauterine devices (IUD). Myovant's treatment, on the other hand, is a non-invasive pill designed to shrink the fibroids. It faces some competition from a new drug from AbbVie (ABBV), but Myovant's entry could have a long runway for future growth and sales considering the market for treating uterine fibroids is still young.
Better still is that Relugolix has even more potential to shrink tumors. Myovant recently partnered with Pfizer (PFE) to run trials testing a combination of the drug for its contraceptive efficacy. Moreover, the compound is also seen as a treatment for endometriosis.
All of this kicks off for Myovant on June 1, when the FDA is expected to hand down its decision on using Relugolix to treat uterine fibroids – a ruling that could have an impact on the biotech stock.

Biogen
- Market value: $42.3 billion
- Timeline to watch: June 2021
Truth be told, monster biotech Biogen (BIIB, $281.01) has had a long fight with Alzheimer's medications. The affliction is incredibly hard to fight and there currently are not any medications designed to really treat it.
BIIB hopes to change that, but the road has been bumpy.
Biogen originally stopped trials on aducanumab back in 2019 after early data suggested the drug would not "meet the primary endpoints." BIIB did a U-turn on the treatment later that year, saying it would submit it to the FDA after more trials. The bumps have continued to this day.
In November, an FDA advisory panel voted that they couldn't judge effectiveness of aducanumab based on the one trial submitted. Worse still was that several doctors in the FDA panel have expressed concerns about approving the potential Alzheimer's treatment through a Journal of the American Medical Association (JAMA) article.
The problem for Biogen is that it needs aducanumab approval to find growth over the long term.
Biogen does mint cash through its blockbuster multiple sclerosis (MS) drug, Tecfidera. Sales here clocked in at more than $2.6 billion last year. The issue is that new generic drugs are starting to eat away at Tecfidera revenues in a big way. Meanwhile, new MS treatments under Biogen's own umbrella, like Vumerity, have failed to live up to expectations. All in all, total revenues for BIIB slipped about 6% in 2020 versus 2019's numbers. BIIB already guided to lower revenues this year, as well.
Now, Biogen isn't in danger of going out of business. But without approval for the Alzheimer's drug, growth prospects could be limited for the near future. That's why the green light is so critical for the biotech stock on June 7, when the FDA decision on aducanumab is due.

Vertex Pharmaceuticals
- Market value: $55.6 billion
- Timeline to watch: June 2021
When it comes to cystic fibrosis (CF) treatments, Vertex Pharmaceuticals (VRTX, $214.65) is the leader hands down. Three of its FDA-approved drugs – Kalydeco, Orkambi, and Symdeko – are some of the top treatments for the disease. As such, they're cash cows and drive VRTX's hefty revenues. Last quarter alone, the drugmaker managed to record revenues of over $1.72 billion. That was a 14% increase versus the first quarter of 2020.
Now, Vertex has several other non-cystic fibrosis medications in the works. But CF treatments will drive the show until about 2030 when patents start to expire. VRTX has been successful in pivoting those medicines into new combo therapies and indications. Trikafta, for example, brought in more than $3.9 billion in first-year sales in 2020.
More sales could be on the horizon. On June 8, the FDA will rule on allowing Vertex to use Trikafta in children with cystic fibrosis ages 6 through 11 with certain mutations. Again, this is an untapped market that will allow VRTX to maintain its dominance in cystic fibrosis treatments. Better still, these are the sort of incremental sales that have contributed to VRTX's long-term earnings growth.
More importantly, the approval will provide Vertex with even more cash to fund its non-CF operations and development. And dare I say it, possibly pay a dividend, too. Last quarter, it ended with $6.9 billion in cash on its balance sheet.
In the end, adding another notch on its belt will allow Vertex to generate even more cash for shareholders – and potentially provide a boost for the biotech stock.

Blueprint Medicines
- Market value: $5.5 billion
- Timeline to watch: June 2021
Thinking "small" has allowed Blueprint Medicines (BPMC, $93.54) to win over the last year or so.
BPMC has focused its research on so-called small-molecule drugs. Unlike regular biologic therapies, small-molecule drugs are more stable. This allows them to be taken as pills rather than through injections. Most biologic drugs come in injectable forms and must be administered by a doctor, nurse or other medical professional. For those drugs that require ongoing and repeat doses, this can be a hassle for many patients.
Given this, small-molecule drugs offer better accessibility and ongoing treatments.
Blueprint Medicines has already scored two approvals – Gavreto and Ayvakit – for certain types of lung and stomach cancers. With the rarity of those cancers, price tags for the medicines are sky high.
Total revenues for BPMC's first quarter of 2021 came in at $21.6 million. While that may seem small, this is a huge 250% gain in sales versus the first quarter of 2020. Ayvakit drove the show, bringing in $7.1 million in net product revenue over the three-month period.
Which is why the additional approval of Ayvakit on June 16 could be a major winner for BPMC.
Blueprint Medicines is looking to use Ayvakit to treat advanced systemic mastocytosis (SM), which is a rare condition caused by the accumulation of mast cells in the body's organs. This leads to potentially debilitating and life-threatening symptoms.
Already, BPMC's treatment has received breakthrough designation status from the FDA. This would allow the company to hold patents for longer and move more quickly through the approval process.
It also could mean plenty of surging revenue. With Ayvakit already causing a major boost to Blueprint Medicines's sales in a short amount of time, any additional wins for the drug should be a windfall – and a positive catalyst for the biotech stock.
Aaron Levitt was long BMY as of this writing.
Profit and prosper with the best of Kiplinger's advice on investing, taxes, retirement, personal finance and much more. Delivered daily. Enter your email in the box and click Sign Me Up.
-
 Dow Adds 1,206 Points to Top 50,000: Stock Market Today
Dow Adds 1,206 Points to Top 50,000: Stock Market TodayThe S&P 500 and Nasdaq also had strong finishes to a volatile week, with beaten-down tech stocks outperforming.
-
 Ask the Tax Editor: Federal Income Tax Deductions
Ask the Tax Editor: Federal Income Tax DeductionsAsk the Editor In this week's Ask the Editor Q&A, Joy Taylor answers questions on federal income tax deductions
-
 States With No-Fault Car Insurance Laws (and How No-Fault Car Insurance Works)
States With No-Fault Car Insurance Laws (and How No-Fault Car Insurance Works)A breakdown of the confusing rules around no-fault car insurance in every state where it exists.
-
 Stocks Struggle Ahead of November Jobs Report: Stock Market Today
Stocks Struggle Ahead of November Jobs Report: Stock Market TodayOracle and Broadcom continued to fall, while market participants looked ahead to Tuesday's jobs report.
-
 The 24 Cheapest Places To Retire in the US
The 24 Cheapest Places To Retire in the USWhen you're trying to balance a fixed income with an enjoyable retirement, the cost of living is a crucial factor to consider. Is your city the best?
-
 15 Cancer Drugs Are in Short Supply, FDA reports: The Kiplinger Letter
15 Cancer Drugs Are in Short Supply, FDA reports: The Kiplinger LetterThe Kiplinger Letter The U.S. is working to address cancer drug shortages caused by manufacturing and supply chain woes.
-
 What Is the Nasdaq?
What Is the Nasdaq?The Nasdaq Composite is one of the most widely followed indexes on Wall Street, and is used to measure the performance of the stock market.
-
 5 Stocks to Sell or Avoid Now
5 Stocks to Sell or Avoid Nowstocks to sell In a difficult market like this, weak positions can get even weaker. Wall Street analysts believe these five stocks should be near the front of your sell list.
-
 How Cytonics' Developed a Revolutionary Treatment for Osteoarthritis, Solving a $240B Problem
How Cytonics' Developed a Revolutionary Treatment for Osteoarthritis, Solving a $240B ProblemSponsored Sponsored Content by Cytonics
-
 Weed Legalization in Florida Gains Ground: This Week in Cannabis Investing
Weed Legalization in Florida Gains Ground: This Week in Cannabis InvestingEnough signatures have been gathered to take recreational weed legalization efforts in Florida to the next step.
-
 Best Stocks for Rising Interest Rates
Best Stocks for Rising Interest Ratesstocks The Federal Reserve has been aggressive in its rate hiking, and there's a chance it's not done yet. Here are eight of the best stocks for rising interest rates.
