How Cytonics' Developed a Revolutionary Treatment for Osteoarthritis, Solving a $240B Problem
Sponsored Content by Cytonics
More than 600 million people worldwide suffer from osteoarthritis (OA), a debilitating joint disease that destroys mobility and quality of life. And for a long time, painkillers and corticosteroids were among the only “treatments” available for this joint-degrading disease.
Then Cytonics came along and developed a way to target the disease at the molecular level.
Their flagship treatment, based on a naturally-occurring blood protein called Alpha-2-Macroglobulin, has treated over 8,000 people so far. Now, they are developing an even more potent and accessible version of it through FDA trials – opening their company to investors meanwhile (you can learn about Cytonics’ investment opportunity here).
Pharma has been desperately trying to develop a drug for osteoarthritis for the last 30 years, and has been unable to do so due to their poor understanding of the molecular underpinnings of the disease. Cytonics has solved this problem, which could mean a huge opportunity for investors.
Cytonics Has Treated 8,000 Patients And Counting
This company has already built a strong track record thus far, starting with their FDA-approved Autologous Protease Inhibitor Concentrate (APIC) therapy. APIC is a device that purifies A2M from a patient’s blood, which can then be injected into the ailing joint.
This was a breakthrough that proved the therapeutic power of A2M in treating cartilage damage in arthritic joints, and while they treated thousands with the APIC therapy, there were some kinks to iron out. APIC injections weren’t cost-effective enough to meet demand from millions struggling with the disease. Due to the fact that the A2M must be purified out of the patient’s blood, the procedure was also a long, tedious process for both doctors and patients. Also, there is patient-to-patient variability in the amount of A2M found in each individual’s bloodstream, meaning that the treatment could be more effective for some people than others. A simpler, more effective treatment that could be produced on an industrial scale and distributed globally was needed.
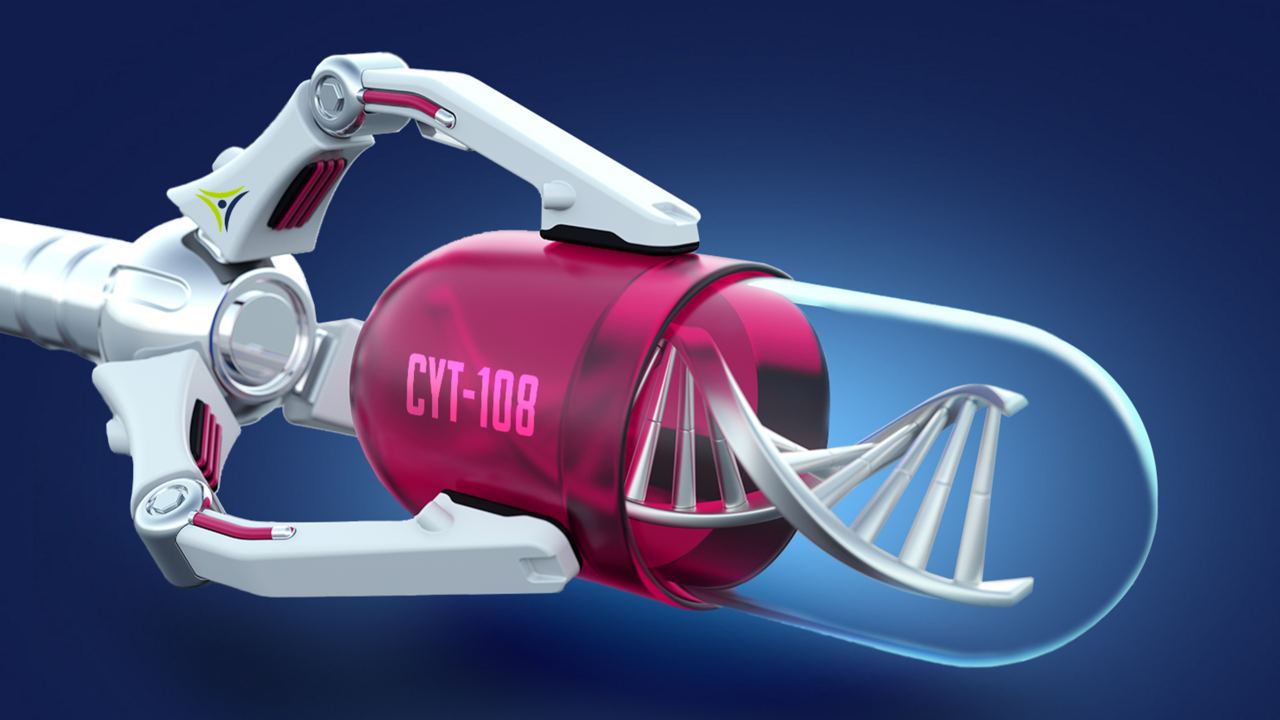
Cytonics rose to this challenge with the development of “CYT-108”, their genetically-modified, synthetic variant of A2M that is 4x more potent than the natural A2M found in blood. If approved by the FDA, CYT-108 will be capable of production on an industrial scale and global distribution as an “off-the-shelf” pharmaceutical that only requires a single injection and no blood preparation.
Not only that, but CYT-108 has the potential to be the first and only “true” therapy for osteoarthritis that addresses the root cause of the disease instead of merely masking the symptoms. This will disrupt an enormous market that is growing as the global population rapidly ages and demands a higher quality of life.
Cytonics’ $240B Market
As one of the largest generations enters retirement, 25% of adults in the U.S. are projected to suffer from osteoarthritis by 2030.
But, believe it or not, this disease can affect just about anyone – old, young, active or not. Before Cytonics, these patients were spending $240B annually on painkillers and corticosteroids to treat the symptoms of their osteoarthritis.
All of that money has the potential to move towards a real treatment for the disease that is affordable, accessible, and can actually stop the destruction of cartilage and promote regeneration. Cytonics achieving FDA approval for CYT-108 would be a big step in that direction.
Cytonics Is Getting Ready To Scale
The clinical and commercial success of Cytonics’ APIC therapy is a testament to the therapeutic power of A2M as a treatment for osteoarthritis. But the limitations of the APIC therapy necessitate a superior technology that overcomes the tedious nature of harvesting A2M from patients’ blood and provides patients with an off-the-shelf pharmaceutical that is highly potent and easily accessible. CYT-108 has the potential to do both.
Instead of long appointments in doctors’ offices, patients could access an even more potent treatment directly from the pharmacy. Also, medical insurance payers will certainly take note of a therapy that reduces the need for total joint replacement, which will rapidly scale CYT-108 into the Medicare marketplace.
With CYT-108 rapidly approaching the Phase 1 human study of the FDA-approval process, there is no better time to invest in Cytonics before they hit this next major milestone.
Learn more about becoming a Cytonics shareholder here.
Equity crowdfunding investments in private placements, and start-up investments in particular, are speculative and involve a high degree of risk and those investors who cannot afford to lose their entire investment should not invest in start-ups. Companies seeking startup investment through equity crowdfunding tend to be in earlier stages of development and their business model, products and services may not yet be fully developed, operational or tested in the public marketplace. There is no guarantee that the stated valuation and other terms are accurate or in agreement with the market or industry valuations. Further, investors may receive illiquid and/or restricted stock that may be subject to holding period requirements and/or liquidity concerns. Kiplinger may receive monetary compensation by the issuer, or its agency, for publicizing the offering of the issuer’s securities. Kiplinger and the issuer of this offering make no promises, representations, warranties or guarantees that any of the services will result in a profit or will not result in a loss.
Profit and prosper with the best of Kiplinger's advice on investing, taxes, retirement, personal finance and much more. Delivered daily. Enter your email in the box and click Sign Me Up.
This content is part of a paid partnership
-
 How Much It Costs to Host a Super Bowl Party in 2026
How Much It Costs to Host a Super Bowl Party in 2026Hosting a Super Bowl party in 2026 could cost you. Here's a breakdown of food, drink and entertainment costs — plus ways to save.
-
 3 Reasons to Use a 5-Year CD As You Approach Retirement
3 Reasons to Use a 5-Year CD As You Approach RetirementA five-year CD can help you reach other milestones as you approach retirement.
-
 Your Adult Kids Are Doing Fine. Is It Time To Spend Some of Their Inheritance?
Your Adult Kids Are Doing Fine. Is It Time To Spend Some of Their Inheritance?If your kids are successful, do they need an inheritance? Ask yourself these four questions before passing down another dollar.
-
 15 Cancer Drugs Are in Short Supply, FDA reports: The Kiplinger Letter
15 Cancer Drugs Are in Short Supply, FDA reports: The Kiplinger LetterThe Kiplinger Letter The U.S. is working to address cancer drug shortages caused by manufacturing and supply chain woes.
-
 Weed Legalization in Florida Gains Ground: This Week in Cannabis Investing
Weed Legalization in Florida Gains Ground: This Week in Cannabis InvestingEnough signatures have been gathered to take recreational weed legalization efforts in Florida to the next step.
-
 8 Biotech Stocks With Major Catalysts on the Horizon
8 Biotech Stocks With Major Catalysts on the Horizoninvesting Biotech stocks frequently make big moves on a single trial result or FDA ruling. These 8 drugmakers are worth watching amid important events on deck this summer.
-
 Save the Date: 11 Biotech Stocks to Put on Your Radar
Save the Date: 11 Biotech Stocks to Put on Your Radarstocks Here are 11 biotech stocks and pharmaceutical companies to watch over the next few months.
-
 10 Blockbuster Drugs of the Future
10 Blockbuster Drugs of the Futureinvesting The pharmaceutical industry occasionally hits a home run by creating a drug that generates sales in excess of $1 billion per year – the watermark for what constitutes a so-called “blockbuster.” These heavy-hitters are a rarity relative to the number of drugs that begin development but never really go anywhere.
-
 7 Stocks Under $10 Worth Buying
7 Stocks Under $10 Worth Buyinginvesting When a stock costs less than dinner at a fast-food joint, there’s almost always a reason.